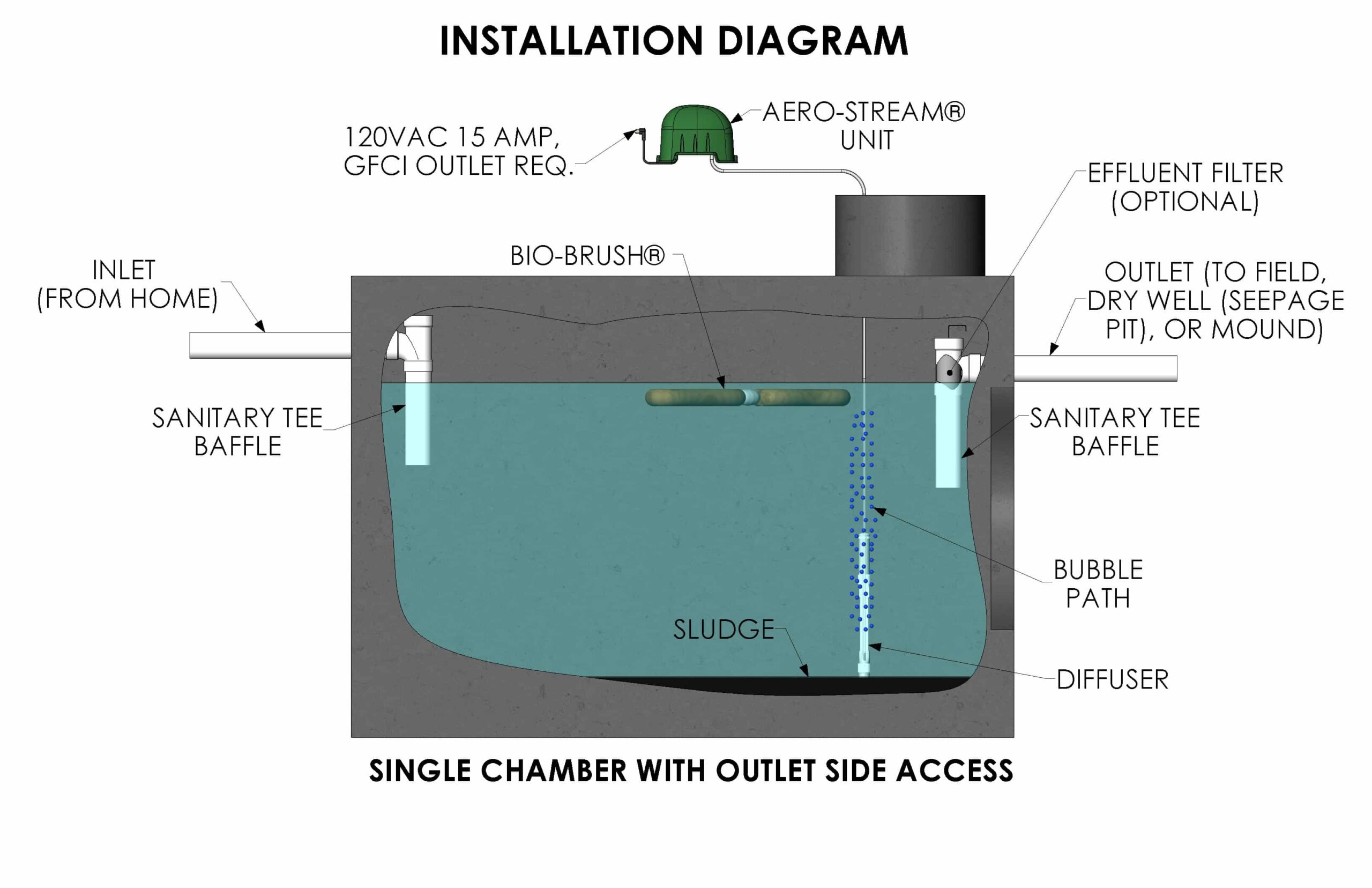
You may be familiar with the different methods of treating water, but the actual science behind each process can be mystifying. Water purification systems are designed for specific types of water, which is important to keep in mind as you consider a home, garden, or laboratory setting.

Carbon
When water passes through a carbon filter, the activated carbon granules will reach out and grab the offending contaminants– the chemical equivalent of an underwater police lineup. Your basic carbon filter will haul Chlorine off to the slammer and improve the overall taste of the water. However, if you have a more effective carbon filter, it will perform a mass arrest of several “bad guys” — contaminants such as Asbestos, Lead, Mercury and Volatile Organic Compounds (VOCs).

UV (Ultraviolet)
Ultraviolet (UV) technology is subtler than carbon filtering—it acts more like an undercover cop with a specific weapon of choice: Light. An Ultraviolet (UV) disinfection system transfers electromagnetic energy from a mercury arc lamp to an organism. When the UV radiation infiltrates the cells of the organism, it effectively destroys the cell’s ability to reproduce. Though UV light slays bacteria and other microorganisms, it cannot remove chemical contaminants. One major advantage of UV technology is that it’s not harmful to humans or aquatic life.

Ozone
Ozone water treatment uses colorless Ozone gas, a powerful oxidant, to eliminate organic and inorganic compounds in water. The Ozone gas acts like a self-destructing message out of Mission Impossible, but instead of lighting on fire, the gas spontaneously decomposes in the water treatment stage via an oxidation process. Ozone disinfection is more effective against bacteria and viruses than chlorination, but it is not effective in removing chemical contaminants. Ozone technology helps to restore the water’s crisp, mountain-stream taste by removing odors during the treatment process. Ozone is typically used in combination with other filtering methods.
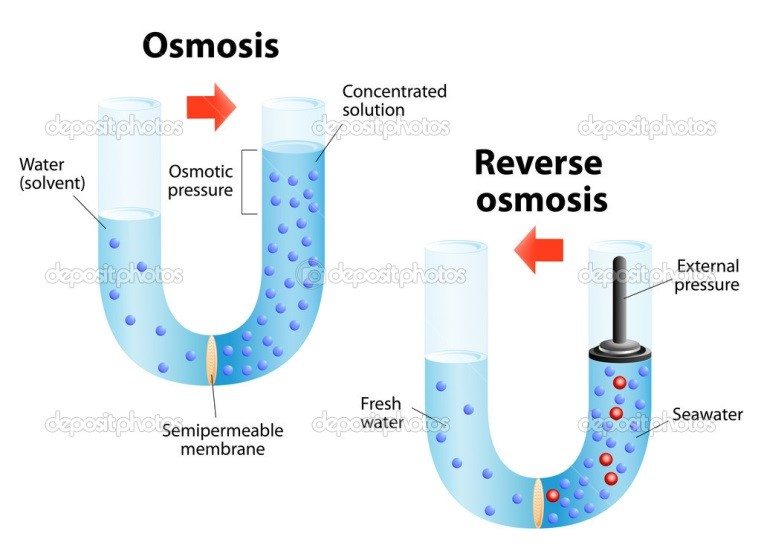
Reverse Osmosis
Reverse Osmosis treatment, typically used for small amounts of drinking water, involves the use of a semi-permeable membrane, which allows certain molecules or ions to pass through. The semi-permeable membrane acts like a backstage bouncer at a rock concert, allowing certain people (molecules) to pass through the gate. In osmosis, water molecules pass through membranes that separate a diluted solution from a concentrated solution. This separation places the molecules on each side of a membrane, which generates osmotic pressure. When pressure is added to the higher contaminant solution, the natural flow is reversed. The reversal results in a higher concentration of contaminated solution, producing purified water in the other solution.

Distillation
A heat source “zaps”, i.e., vaporizes the water during the distillation treatment process, separating the pure water molecules from contaminants. The water is heated until it reaches its boiling point and begins the evaporation process. The temperature is kept constant, which allows the vaporization process to continue. Contaminants with a higher boiling point than water will not evaporate; hence the evaporated water is captured and shuttled to another container. This process removes most minerals, bacteria, viruses, and any chemicals that have a higher boiling point than water. Distilled water is often used in science labs and for printing presses, due to its mineral-free status. The only hitch: Distillation fails to remove chlorine or other VOCs.

Water Softeners
Ever wonder about the difference between Hard Water vs. Soft Water? Water Softening is the removal of calcium, magnesium, and other metals in hard water (water with high mineral content). Soft water interacts better with soaps, and is gentler on your plumbing. Metal ions in hard water, if left untreated, can lead to a buildup of lime scale- a chalky deposit that can lead to corrosion or plumbing issues. Water softeners use an ion exchange process to lower the levels of minerals such as calcium, magnesium, barium and radium. What’s the downside? Your water may be higher in sodium. Check with your medical provider prior to using water softeners at home.
All water purification methods have pros and cons and are used differently depending on the specific need. Researching the type of water in your home, office, or travel area will help you to determine what type of purification system you need.